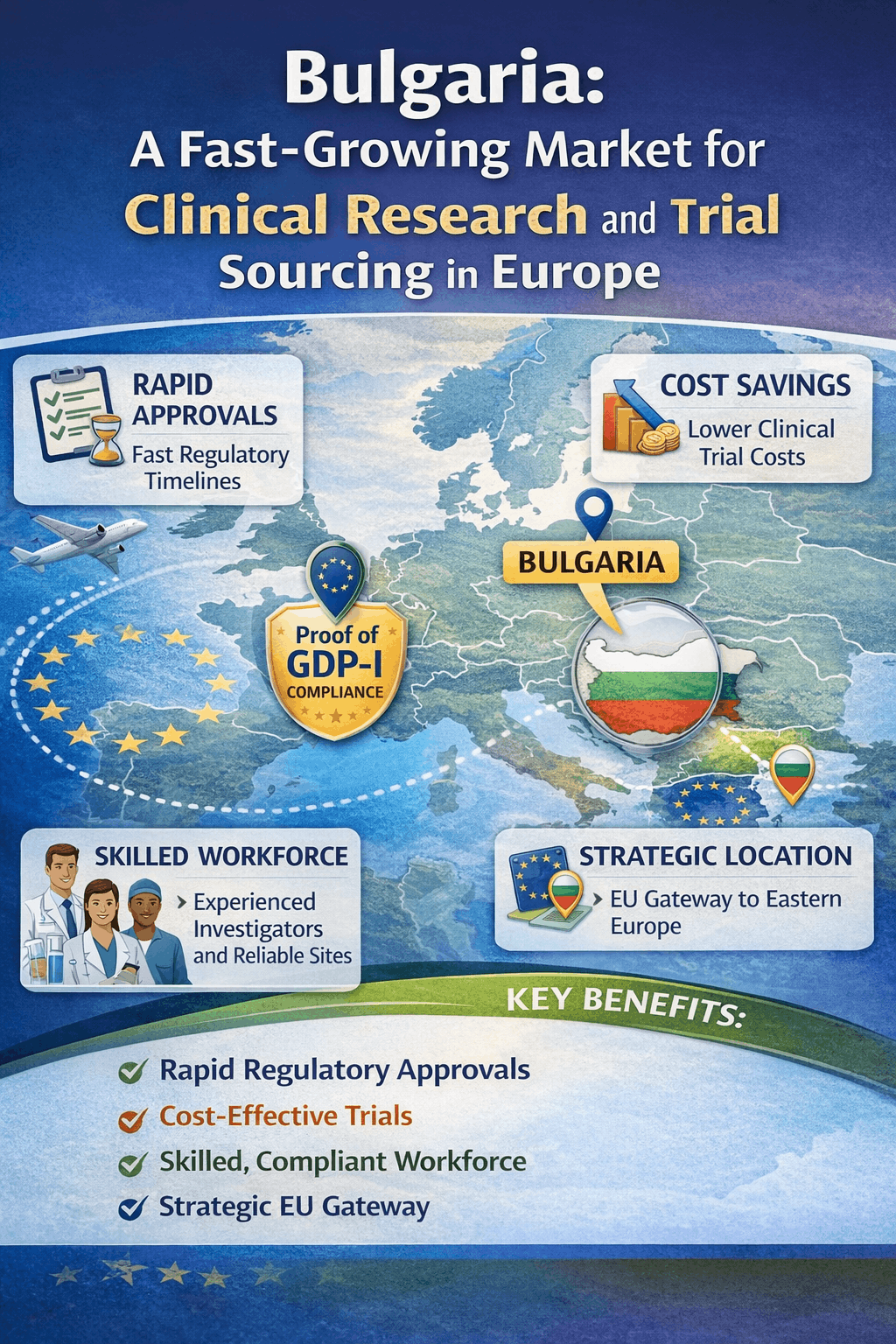
Bulgaria has became Europe’s fast-growing markets for medicine research. Although historically disregarded in favour of Western European markets, Bulgaria is now known for its cost-effective research environment, skilled medical workforce, and strong regulatory alignment with the European Union.
As of December 2025, Bulgaria’s drug research market is estimated to exceed approximately USD 360 million, with the country ranking among the top 20 global locations for active trials. This expansion reflects growing sponsor confidence in Bulgaria’s capacity to provide high-quality data, effective patient recruitment, and dependable operational execution—making it a desirable site for RLD sourcing as well as comparator drug sourcing activities.
Bulgaria’s Patient Population and Demographic Advantage
Bulgaria has a population of approximately 6.5 million as of December 2025, and its demographics are gradually getting older. Bulgaria now has one of the oldest populations in the EU, with a median age of over 45.
This demographic trend creates a strong foundation for research programs in therapeutic areas such as:
- Oncology
- Metabolic disorders
- Respiratory diseases
- Cardiovascular diseases
- Neurological and age-related conditions
Non-communicable diseases are dominating Bulgaria’s disease burden. Cardiovascular diseases account for almost 60% of all mortality, followed by cancer at about 18%. These make Bulgaria highly relevant for late-phase trials, real-world evidence generation, and comparator products in BE /BA study targeting chronic and life-threatening diseases.
Lifestyle risk factors—such as high alcohol consumption, smoking prevalence, and sedentary behavior, further contribute to patient availability across multiple indications.
Therapeutic Trials Landscape in Bulgaria:
By the end of 2024, Bulgaria had more than 800 active therapeutic trials, and in 2025, the number of newly approved trials continues to average 200–220 studies per year. Over 13,000 patients now participate annually in medicine study across the country.
This sustained activity has increased Bulgaria’s role as a strategic location for:
- Phase II–IV studies
- Multinational and multi-center studies
- Bioequivalence (BE) and comparative studies
- RLD sourcing within the EU
The country’s consistency in trial execution and data quality has made it increasingly attractive to global sponsors and clinical trial sourcing companies.
Why Bulgaria Is a Preferred Destination for Medical Research Study:
Bulgaria’s increasing significance in the European medical research ecosystem can be explained by a number of structural advantages:
- Highly Qualified Medical Workforce: Bulgaria has the highest physician-to-population ratios in Eastern Europe, with a strong base of English-speaking investigators experienced in international protocols.
- Cost-Effective Trial Execution: Operational and labor costs remain 30 to 50% lower than Western Europe. This allows sponsors to optimize budgets without compromising quality.
- Fast Patient Recruitment: Bulgaria benefits from centralized healthcare access and high physician influence. This enable faster enrollment and strong patient retention.
- Strategic EU Location: As an EU member state, Bulgaria provides seamless regulatory compatibility for clinical trial sourcing, comparator drug sourcing, and cross-border trial supply.
Regulatory Environment: EU-Compliant and Sponsor-Friendly
Bulgaria operates fully under the EU CTR 536/2014 and the CTIS.
Key Regulatory Updates:
- Accelerated Approval Timelines: The Bulgarian Drug Agency (BDA) now targets review timelines of 30 to 35 days. This place it among the faster EU regulators.
- Mandatory CTIS Transition: As of January 31, 2025, all trials approved under legacy national systems must be fully transitioned to CTIS.
- Streamlined Ethics Review: Harmonized ethics committee processes have reduced duplication and shortened study start-up timelines.
Site Feasibility and Research Infrastructure:
The country offers a dense and capable medical research infrastructure:
- 1,000+ hospitals and study sites, with strong concentration in Sofia, Varna, Plovdiv, and Burgas
- Investigators with huge multinational trial experience
- Modern diagnostic and laboratory facilities aligned with GCP and GDP standards
While rural sites may face limitations related to infrastructure, urban centers consistently meet international sponsor expectations for complex trials and interventional studies.
Patient Recruitment Strengths and Considerations:
Why Patient Recruitment Works Well in Bulgaria?
- Large treatment-naïve patient population
- High trust in physicians, leading to strong enrollment compliance
- Lower dropout rates compared to Western Europe
- Growing use of digital tools and hospital registries to support recruitment
Challenges to Acknowledge
- Less public awareness of drug research
- Strict rules governing trial advertising and patient outreach
Despite all these challenges, Bulgaria remains one of the most efficient EU markets for patient enrollment and retention.
Bulgaria’s Role in RLD Sourcing and Comparator Drug Supply
Apart from trial execution, Bulgaria is increasingly leveraged for reference listed drug sourcing, including:
- Comparator drug sourcing
- Access to EU-authorized reference medicines
- GDP-compliant storage and distribution
- Reliable batch traceability for regulatory submissions
For sponsors conducting global studies, Bulgaria offers a strategic base for sourcing comparator products in drug trials, particularly when consistency, documentation, and EU compliance are required.
Final Perspective: Why Bulgaria Belongs on Every Sponsor’s Shortlist
Bulgaria has firmly positioned itself as a growing market for therapeutic research in Europe. With a skilled medical workforce, efficient regulatory processes, competitive costs, and expanding expertise in reference listed drug sourcing and comparator sourcing, the country offers a compelling value proposition for global sponsors.
For organizations looking for reliable trial execution, efficient patient recruitment, and dependable sourcing, Bulgaria represents a balanced blend of quality, speed, and compliance.
Ikris Pharma– Your Trusted RLD Sourcing Company in Bulgaria
Ikris Pharma provides clinical trial management services in Bulgaria. The company support pharma and biotechnology companies throughout the study lifecycle. We have a lot of experience in innovator drug sourcing, ensuring uninterrupted access to required comparator products. By coordinating compliant supply chains and regulatory documentation, we help manufacturers and sponsors conduct their medical research smoothly without delays or disruptions.
Get in touch with Ikris Pharma to ensure uninterrupted innovator drug or RLD supply for your studies.
Frequently Asked Questions (FAQs):
1. Why is Bulgaria becoming a popular location for BE/BA study in Europe?
Bulgaria offers EU-compliant regulations, quick approval timelines, experienced investigators, and affordable operational costs compared to Western Europe. These factors make it attractive for sponsors looking for efficient and high-quality medical research execution.
2. How does Bulgaria support innovator drug sourcing?
As an important EU member state, Bulgaria provides access to authorized medicines, GDP-compliant logistics, and full batch traceability. This makes it suitable for innovator drug sourcing, including comparator drug sourcing for multinational studies.
3. What types of trials are commonly conducted in Bulgaria?
Bulgaria hosts Phase II to Phase IV trials, oncology and cardiology research, and BE studies requiring comparator products in BA/BE studies, supported by experienced investigators and research sites.
4. Can Bulgaria be used as a base for comparator sourcing for BA/BE studies?
Yes. Bulgaria is increasingly used as a strategic base for innovator sourcing for drug development because of its EU regulatory alignment, availability of reference medicines, and reliable GDP-compliant supply chains.
References:
- CTIS – European Commission
- Bulgarian Drug Agency (BDA)
- World Health Organization (WHO) – Research and Non-Communicable Diseases
- Eurostat – Population and Health Statistics for Bulgaria
- International Federation of Pharmaceutical Manufacturers & Associations (IFPMA)
- OECD Health Statistics – Disease Burden and Healthcare Data
(These references support regulatory accuracy, population data, and medical research credibility.)