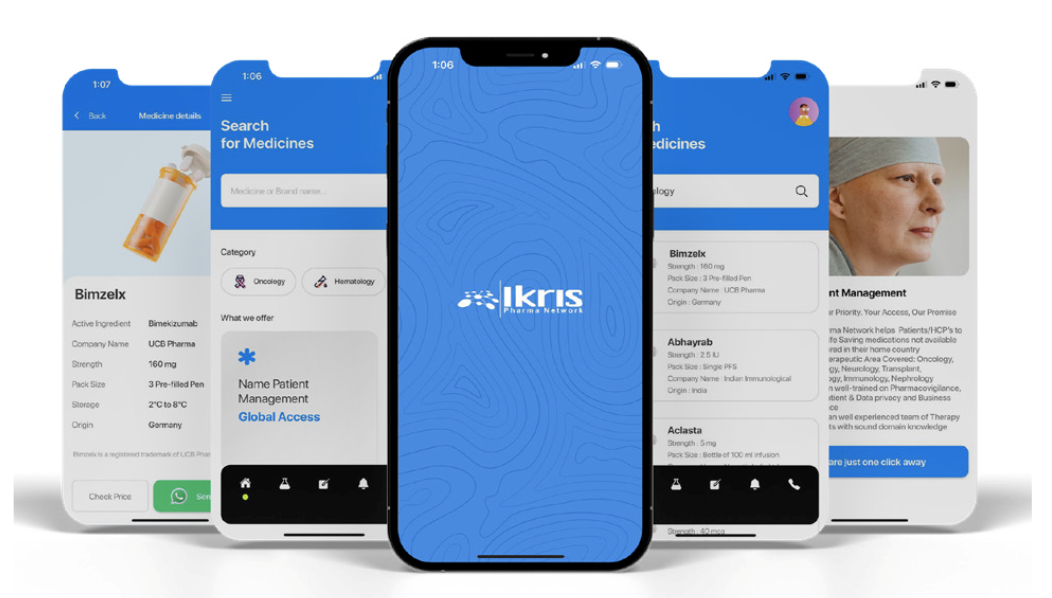
Compliance at the Core of Every Supply
Pharmaceutical sourcing and distribution aligned with EU GDP principles and institutional procurement requirements.

Compliance is the foundation of our operations. Ikris Pharma Network aligns its processes with EU GDP principles and procurement expectations.