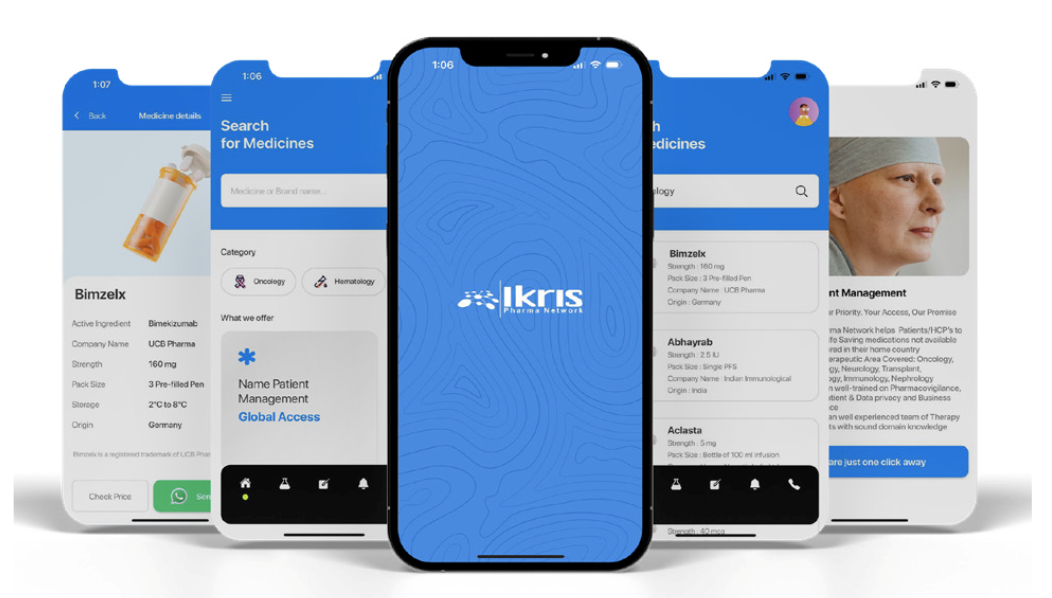
Access to Medicines Beyond Standard Approvals
Specialised support for unlicensed medicines and Named Patient Programs under European special access frameworks.

Ikris Pharma Network supports healthcare providers with access to unlicensed and unapproved medicines under European special access and Named Patient frameworks.
Our team supports compliant sourcing for: